Search Results
Results for: 'water molecule'
Non-polar compounds - insolubility
By: HWC, Views: 6686
• A non-polar molecule has uniform distribution of electrons. • Non-polar compounds like fatty acids in lipids have a high proportion of carbon and hydrogen. • Lipids possess no charge or partial charge. • Lipids are not attracted to water molecules. • Lipids are not soluble in...
Properties of water -structure of water and polarity (Ionized and polar compounds)
By: HWC, Views: 6823
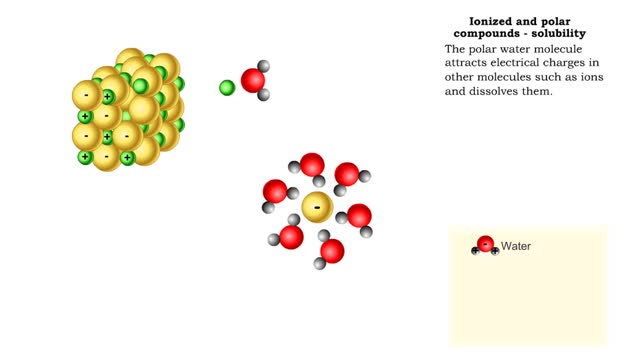
■ Water transports most of the molecules in the body. ■ The structure of a water molecule allows it to dissolve other molecules. ■ Shared electrons spend more time near the oxygen atom. ■ Oxygen end has a partial negative charge. ■ Hydrogen ends have a partial positive charge....
Condensation and Hydrolysis Animation
By: HWC, Views: 527
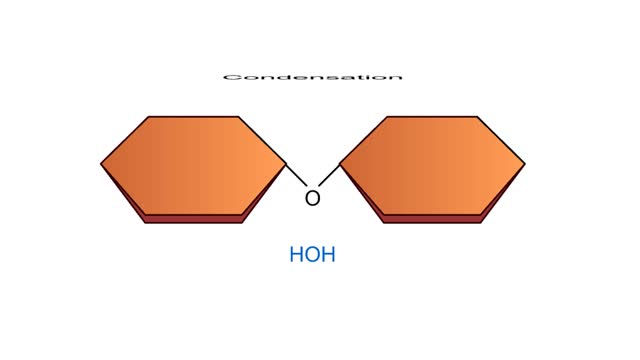
A condensation reaction joins two molecules together to form one larger molecule. An enzyme removes a hydroxyl group from one molecule and a hydrogen atom from another, then speeds the formation of a bond between the two molecules at their exposed sites. Typically the discarded atoms join t...
What are Strong & Weak Acids and How they're different?
By: HWC, Views: 5483
Let's consider the changes that take place when hydrogen chloride, HCI, is added to water. You will need to recognize space-filling models of HCI molecules, hydronium ions (H30+), chloride ions (C11, and water molecules (H20). They are shown at the right. When HC1 molecules dissolve in water, ...
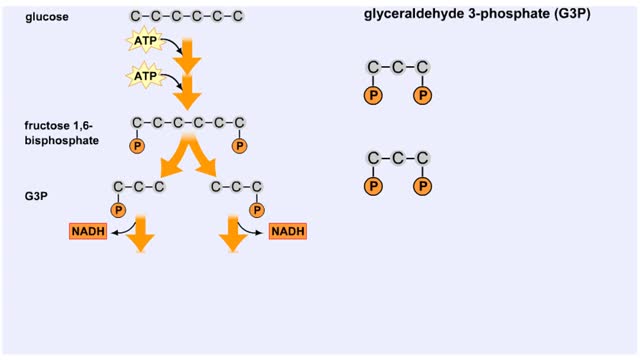
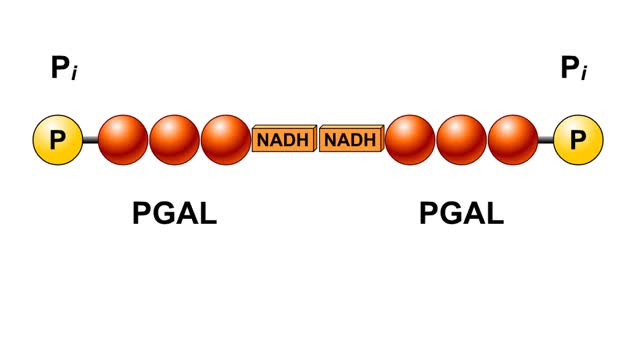
Splitting of Sugar, Oxidation/ Reduction & ATP Generation
By: HWC, Views: 6357
The next reaction shows us the meaning of "glycolysis" or the splitting of glucose. The fructose bisphosphate molecule is split into two molecules each containing 3 carbons as the backbone. FBP is split into two 3-carbon molecules called G3P, or glyceraldehyde 3-phosphate. Notice that the phos...
By: HWC, Views: 570
In glycolysis, a six-carbon glucose molecule is split into two three-carbon pyruvate molecules. In this animation, each carbon molecule is represented by a red ball. The end products of glycolysis are two molecules of pyruvate. Glycolysis is the breakdown of glucose into two molecules of ...
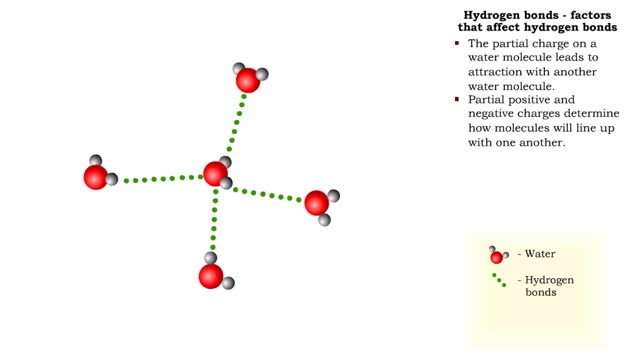
Hydrogen bonds - role in the body
By: HWC, Views: 7010
A hydrogen bond is the electromagnetic attraction between polar molecules in which hydrogen is bound to a larger atom, such as oxygen or nitrogen. This is not a sharing of electrons, as in a covalent bond. Instead, this is an attraction between the positive and negative poles of charged atoms. ...
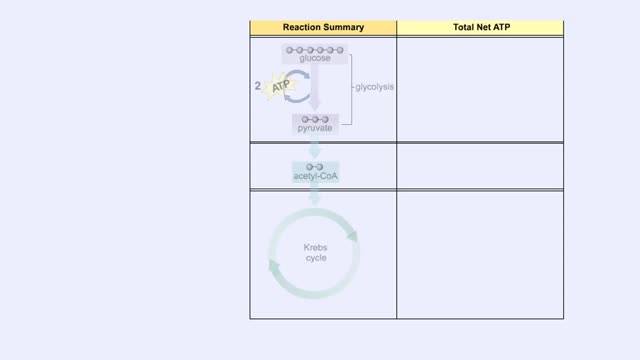
ETC Protein Complexes & Chemiosmosis (Total ATP Production and ATP Synthase)
By: HWC, Views: 6349
You will notice that FADH2 donates two electrons further downstream than NADH. This results in only two protons being pumped across the inner membrane. The final electron acceptor for these transported electrons is oxygen. Oxygen receives these electrons, plus protons from the aqueous matrix. ...
By: HWC, Views: 5458
The slight positive charge of a hydrogen atom in a water molecule can attract an atom with a slight negative charge, such as the nitrogen in a molecule of ammonia. This forms a hydrogen bond between the two atoms. Hydrogen bonds join the two strands of a DNA molecule. Although hydrogen bo...
Advertisement